FAQ
Osteoarthritis is a leading cause of disability not only in the US and around the world. A total joint replacement is the standard of care for older patients, but there are not enough viable solutions for younger patients needing treatment. At CytexOrtho, we have generated significant pre-clinical data, established manufacturing processes, and are now progressing toward a pilot clinical study of our lead product, an acellular implant to restore hip joint health in young patients needing surgical intervention.
We are on a mission to develop regenerative technologies for these patients. This page outlines our path to the clinic and answers some of the frequently asked questions we receive from patients. For the most current updates please sign up for our newsletter!

FAQ
Osteoarthritis is a leading cause of disability not only in the US and around the world. A total joint replacement is the standard of care for older patients, but there are not enough viable solutions for younger patients needing treatment. At CytexOrtho, we have generated significant pre-clinical data, established manufacturing processes, and are now progressing toward a pilot clinical study of our lead product, an acellular implant to restore hip joint health in young patients needing surgical intervention.
We are on a mission to develop regenerative technologies for these patients. This page outlines our path to the clinic and answers some of the frequently asked questions we receive from patients. For the most current updates please sign up for our newsletter!

Frequently Asked Questions
What is Osteoarthritis?
Osteoarthritis (OA) is the most common type of arthritis. Cartilage breaks down, which ultimately allows bones under the cartilage to rub against each other. People with OA experience greater pain, fatigue, levels of disability, and activity limitations.
When will CytexOrtho's clinical trials start?
This is the question we get more often than any other. We get calls and emails almost daily from patients and their families asking about our clinical trials, how can they participate, and when our products will finally be available.
While we don’t have a specific date yet, we’re thrilled to announce that we’ve received clearance to conduct a clinical trial of our ReNew™ Hip implant in 2025. We’re busy putting together the details, so stay tuned! (Check out “What’s next for CytexOrtho?” below for a sneak peek!)
Who can participate in the ReNew™ Hip clinical trial?
The following is a list of the full inclusion and exclusion criteria approved by FDA. Eligibility for trial participation will be determined by the physicians performing the surgeries after a medical evaluation. We will provide contact information and instructions for how to request participation just as soon as we can!
In order to be eligible to participate in this study, subjects must meet all of the following criteria:
1. At least 14 years of age to no older than 55 years of age
Subjects 14-21 years of age must have radiographic evidence of epiphyseal closure in the hip joint
2. BMI < 35
3. Failed at least 6 weeks of conservative treatment (e.g., anti-inflammatory pain medications, physical therapy, injections)
4. Duration of symptoms consistent with intra-articular disease (i.e., groin, lateral and/or posterior hip pain) that have persisted for at least 3 months
5. Loss of articular cartilage integrity (~1 – 6 cm2 in area) on the femoral head (confirmed by MRI), without an opposing lesion that can be treated with a single ReNewTM Hip Implant
6. Radiographic assessment with joint space width > 2 mm (verified by x-ray)
7. Meets an acceptable preoperative medical clearance and is free of conditions that would pose excessive operative risk
8. Given consent to participate in the study
9. Able to understand the purpose of the study, his/her role, and is available for follow-up with a 3-year extension:
a. Subject has signed an IRB (Institutional Review Board) approved Informed Consent Form agreeing to participate in the extension study after the nature, scope, and possible consequences of the study have been explained in an understandable form
b. Subject is able to fully understand the purpose of the study, his/her role as a participant in the study, and plans to be available through five years post-operative follow-up
Exclusion Criteria
Subjects who meet any of the following criteria will be excluded from participating in this study:
1. Smoker (< 1 month cessation of smoking)
2. Type 1 or Type 2 Diabetes
3. Systemic Steroid use in past 3 months
4. Coxa plana, coxa magna, or proximal femoral focal deficiency on the femoral head
5. Any acute or chronic condition that would limit the ability of the patient to participate in the study (e.g., COPD, congestive heart failure),
6. Bleeding disorders
7. Current cancer (with the exception of non-melanoma skin cancer)
8. Pregnancy or planning to become pregnant during the study period
9. Active infection or sepsis
10. History of local hip infection
11. Known metastatic or neoplastic disease
12. Conditions that may interfere with implant survival or outcomes (e.g., severe dysplasia)
13. Life expectancy less than 2 years
14. Intra-articular therapy within 3 months of enrollment
15. Inadequate bone stock (as determined by SCORE or MORES assessment) to support the device
16. Femoral heads is:
a. outside of the 46 – 56 mm range in either anteroposterior diameter or lateral diameters
b. an aspherical head deformity
17. Moderate to severe renal insufficiency
18. Emotional or neurological condition that would preempt ability or willingness to participate in the study
19. Above the knee amputation of the contralateral or ipsilateral leg
20. Known allergies to the components of the devices (polycarprolactone)
21. Is a prisoner
Why does the product development process take so long?
Medical product development is a slow process by design, in order to ensure that medical products (i.e. medicines, devices, diagnostics) in the US are safe and work as intended. That is why CytexOrtho and all biomedical products companies go through lengthy and expensive clinical trials.
What is CytexOrtho's product development process?
A quick internet search will reveal a couple of different – and potentially confusing – FDA medical device approval processes. CytexOrtho’s implanted devices fall under what is called an FDA Class III device, and it is further categorized under the premarket approval (PMA) path for brand new products, in contrast to add-ons to existing products. The PMA route is the most rigorous of FDA’s various pathways to market for medical devices.We have provided a timeline outlining the typical PMA stages involved in getting a new device to market to give you an idea of what product development entails from brainstorming to product availability.
What is CytexOrtho working on now?
CytexOrtho’s potential future products are currently being tested in a variety of preclinical testing models. We have published many of our studies to date, and we have also shared these results with FDA. Our data, to date, provide evidence that our implant technology has potential for treating pre-arthritic lesions as well as early- to moderate-stage OA. We are currently getting the agreements with the clinical sites in place to conduct the clinical trial with the ReNew™ hip implant.
View the image on the right to see our current progress in the FDA approval process.
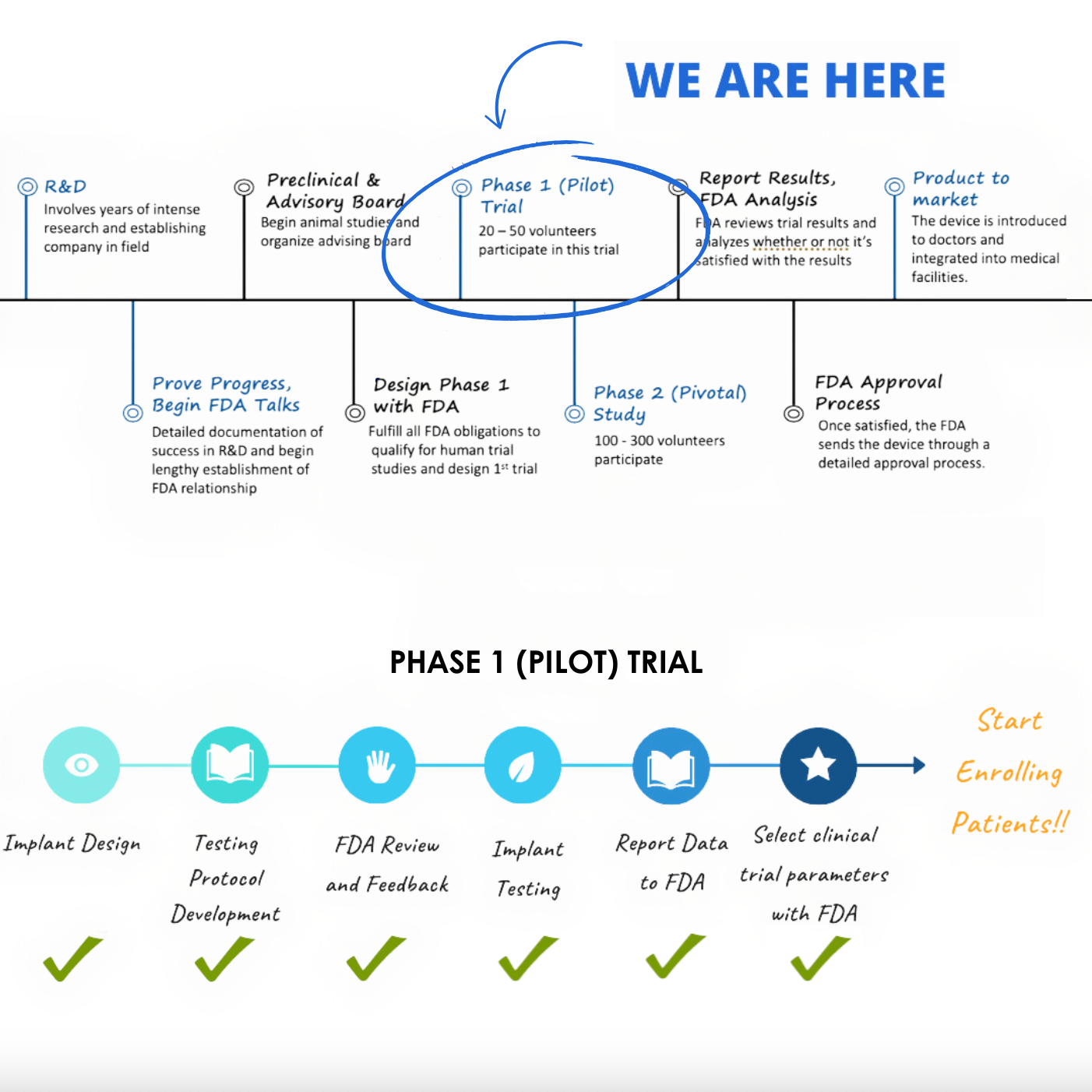
What’s next for CytexOrtho?
Next steps with FDA approval
CytexOrtho has been approved for it’s Phase I clinical trial of the ReNew™ Hip Implant. The trial will enroll up to 15 patients aged 14-55 years with hip disease resulting in loss of articular cartilage integrity on the femoral head. If we are able to meet the endpoints of this initial pilot study, CytexOrtho will conduct advanced clinical trials at clinics around the US at a future date. These later-stage trials, termed pivotal studies, will likely enroll 150 to 250 patients.

Current preparation for clinical trials
With the recent approval by FDA to start a pilot clinical trial, we are in full swing getting the preparations underway! We have approval to conduct the trial at 1 or 2 clinical sites, so the next step is to select these sites and then complete the agreements necessary to begin the trial. This may take several months to do and involves two primary steps: 1) negotiating a clinical trial agreement that defines the costs and terms of conducting the trial and 2) forming an agreement with an Internal Review Board (IRB) that will ensure that safety end points are met. While these agreements are being finalized, we will be working on posting the trial details to clinicaltrials.gov, manufacturing the implants to be used in the trial, and completing the training of the operating surgeons on the final clinical protocols.
Complete details about the trial, including full inclusion and exclusion criteria and contact information for those wishing to participate, will be posted on clinicaltrials.gov, but here are some of the details FDA approved:
- We can enroll up to 15 patients.
- The allowed age range for patients is between 14 and 55 years of age.
- Eligible patients include those who have failed at least six weeks of conservative treatment and have a loss of articular cartilage integrity of approximately 1-6 cm² in area on the femoral head, with a joint space width greater than 2 mm.
We appreciate your patience while we nail down the details. We’re excited to finally bring this game-changing technology to those who need it most – the patients!

