FAQ
Osteoarthritis is a leading cause of disability not only in the US and around the world. A total joint replacement is the standard of care for older patients, but there are not enough viable solutions for younger patients needing treatment. At CytexOrtho, we have generated significant pre-clinical data, established manufacturing processes, and are now progressing toward a pilot clinical study of our lead product, an acellular implant to restore hip joint health in young patients needing surgical intervention.
We are on a mission to develop regenerative technologies for these patients. This page outlines our path to the clinic and answers some of the frequently asked questions we receive from patients. For the most current updates please sign up for our newsletter!

FAQ
Osteoarthritis is a leading cause of disability not only in the US and around the world. A total joint replacement is the standard of care for older patients, but there are not enough viable solutions for younger patients needing treatment. At CytexOrtho, we have generated significant pre-clinical data, established manufacturing processes, and are now progressing toward a pilot clinical study of our lead product, an acellular implant to restore hip joint health in young patients needing surgical intervention.
We are on a mission to develop regenerative technologies for these patients. This page outlines our path to the clinic and answers some of the frequently asked questions we receive from patients. For the most current updates please sign up for our newsletter!

Frequently Asked Questions
What is Osteoarthritis?
Osteoarthritis (OA) is the most common type of arthritis. Cartilage breaks down, which ultimately allows bones under the cartilage to rub against each other. People with OA experience greater pain, fatigue, levels of disability, and activity limitations.
When will CytexOrtho's clinical trials start?
This is the question we get more often than any other. We get calls and emails almost daily from patients and their families asking about our clinical trials, how can they participate, and when our products will finally be available.While we don’t have a date yet, our team has made tremendous advances to get us close to this huge milestone for us. We are happy to report that we have applied for a clinical trial and are awaiting FDA’s decision. If approved, we hope to begin the trial in the summer or fall of 2024.
Why does the product development process take so long?
Medical product development is a slow process by design, in order to ensure that medical products (i.e. medicines, devices, diagnostics) in the US are safe and work as intended. That is why CytexOrtho and all biomedical products companies go through lengthy and expensive clinical trials.
What is CytexOrtho's product development process?
A quick internet search will reveal a couple of different – and potentially confusing – FDA medical device approval processes. CytexOrtho’s implanted devices fall under what is called an FDA Class III device, and it is further categorized under the premarket approval (PMA) path for brand new products, in contrast to add-ons to existing products. The PMA route is the most rigorous of FDA’s various pathways to market for medical devices.We have provided a timeline outlining the typical PMA stages involved in getting a new device to market to give you an idea of what product development entails from brainstorming to product availability.
What is CytexOrtho working on now?
CytexOrtho’s products are currently being tested in in a variety of preclinical testing models required for FDA clinical trials. We have published many of our studies to date, and we have also shared these results with FDA. Our data, to date, provide evidence that our implant technology has potential for treating pre-arthritic lesions as well as early- to moderate-stage OA.
View the image on the right to see out current progress in the FDA approval process.
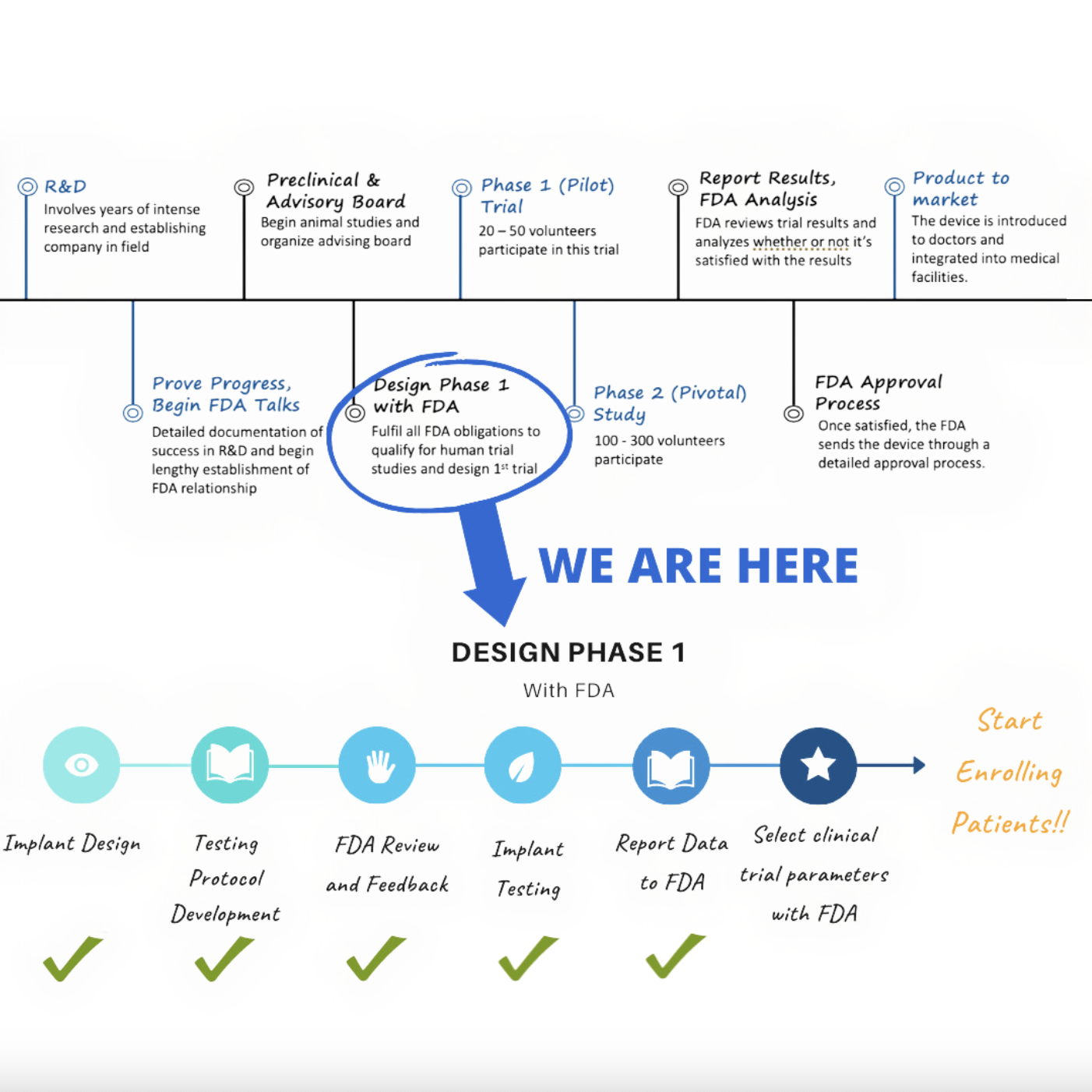
What’s next for CytexOrtho?
Next steps with FDA approval
We are continuing to interact with FDA to make sure the process of filing the documentation supporting the clinical trial is as complete and accurate as possible. This will ensure an efficient path forward to the start the human safety study (sometimes referred to as a Phase I or pilot study).We have filed a formal application called an Investigational Device Exemption or IDE, with the FDA. If approved, we hope to conduct a small pilot study later this year. If we are able to meet the endpoints of the pilot study, CytexOrtho will conduct advanced clinical trials at clinics around the US. These later-stage trials, termed pivotal studies, will likely enroll 200 to 300 patients.

Current preparation for clinical trials
CytexOrtho has a world-renowned Clinical Advisory Board (CAB) consisting of experts in orthopedic surgery and imaging. Input from the CAB has been instrumental for CytexOrtho to plan for our clinical trial, and will keep CytexOrtho focused on the very real challenges these doctors face in treating their patients facing early joint disease.

