Highly anticipated cartilage repair implant from CytexOrtho receives greenlight to move forward with human clinical trials.
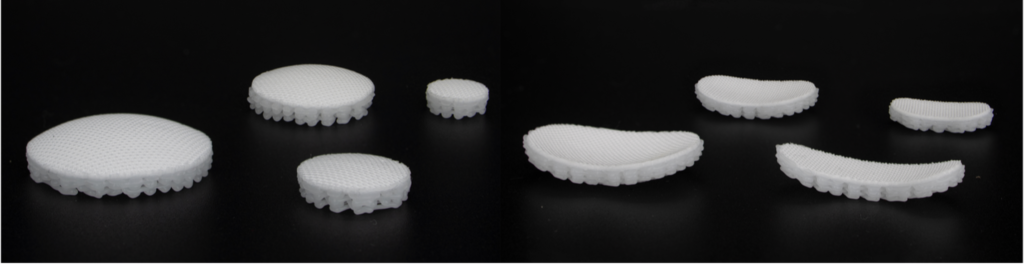
DURHAM, N.C., October 29, 2024 /Issuewire.com/ — CytexOrtho, a pioneer in orthopedic joint preservation, today announced that the U.S. Food and Drug Administration (FDA) has approved its Phase I clinical trial, allowing CytexOrtho to initiate a first-in-human clinical study to evaluate the safety and efficacy of its ReNew™ Hip Implant novel cartilage repair technology.
The proprietary ReNew™ Hip Implant leverages advanced manufacturing techniques to create a unique, highly porous, bioabsorbable device engineered to mimic the properties of healthy articular cartilage. Proven in animal trials, ReNew™ Hip provides immediate structural support, while supporting the body’s own regenerative, healing processes to restore the joint.
CytexOrtho will enroll up to 15 patients aged 14-55 years with hip disease resulting in loss of articular cartilage integrity on the femoral head. The non-randomized, single-arm study will establish initial safety profile and evaluate efficacy in improving pain and function over 12 months. It will follow patients for 60 months post implantation.
“There are over one million American patients under the age of 65 who suffer with chronic hip pain,” said Brad Estes, Ph.D., CEO and Co-founder, CytexOrtho. “Approximately 20 percent of these patients get hip replacements. The rest avoid them because of the high risk of wearing them out and the complications that come with a revision replacement, and instead choose to live with increasingly crippling pain. FDA’s approval of this first human clinical trial brings us closer to delivering new options to the clinic for patients with hip disease. Our ReNew™ Hip Implant aims to change the game by restoring the joint’s anatomical contour with natural tissue regeneration.”
Farshid Guilak, Ph.D., Co-founder of CytexOrtho, Professor of Orthopedic Surgery, and Director of Research, Shriners Children’s – St. Louis, commented, “This IDE approval is backed up by of years of research and development showing success of the ReNew Hip Implant in pre-clinical
animal studies. We believe our approach has the potential to address a significant unmet need in orthopedics.”
“We believe the ReNew™ Hip Implant has tremendous potential to solve a very difficult hip problem where loss of cartilage occurs in the hip joint,” said Jeff Nepple, MD, Assistant Professor of Orthopedics at Washington University School of Medicine. “The ReNew™ Hip implant has a rich pre-clinical validation of its potential to harness the body’s own healing potential to enable cartilage regrowth.”
Thomas Vail, M.D., past Chair of Orthopaedic Surgery at The University of California, San Francisco also added, “Surgeons and patients have long awaited cartilage resurfacing technology, and this FDA approved IDE study is a key step in evaluating the potential for the CytexOrtho ReNew Hip Implant to address that need. If successful, it could offer a path forward for active patients seeking a joint-preserving approach with otherwise limited options.”
About CytexOrtho:
CytexOrtho is dedicated to developing innovative solutions for orthopedic conditions. Founded by leading researchers in the field of cartilage tissue engineering, CytexOrtho aims to revolutionize the treatment of cartilage injuries and osteoarthritis through its proprietary technologies. CytexOrtho’s flagship product, The ReNew™ Hip implant, is a cutting-edge bioabsorbable medical implant that restores the joint for a prolonged, active lifestyle – and if successful, will offer a new treatment option for patients who desperately need solutions. For more information, visit www.cytexortho.com.
Forward-Looking Statements
This press release contains forward-looking statements about CytexOrtho’s expectations, plans and prospects, including statements regarding the clinical development of the ReNew™ Hip Implant. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including the uncertainties inherent in the initiation and conduct of clinical trials. Any forward-looking statements represent CytexOrtho’s views only as of today and should not be relied upon as representing its views as of any subsequent date. CytexOrtho explicitly disclaims any obligation to update any forward-looking statements.
CytexOrtho Media Relations
info@cytexortho.com
