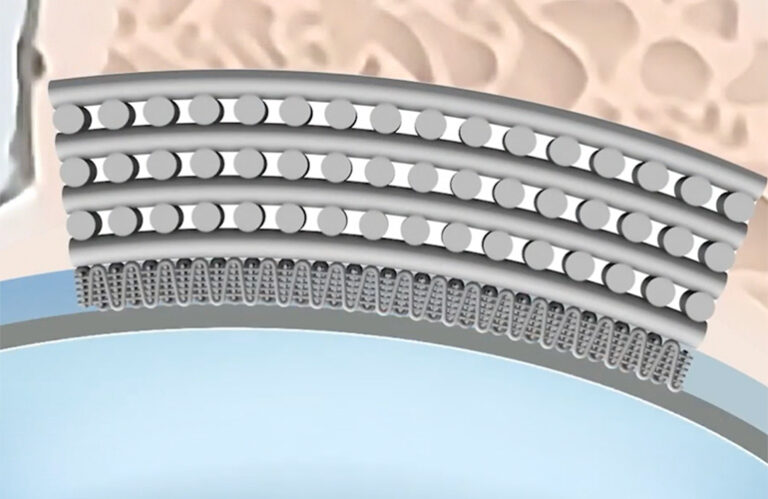
by CytexOrtho | Jun 10, 2024 | In the News
CytexOrtho was highlighted in a recent feature by Medical Design and Outsourcing (MDO) that focused on the diverse applications, industry standards, regulations, and notable challenges associated with bioabsorbable polymers. Since CytexOrtho’s implants are 100%...

by CytexOrtho | May 6, 2024 | In the News
CytexOrtho was thrilled to participate in this year’s Venture Connect! The event offers a platform to ‘gain visibility and potential financial backing while championing the great innovation emerging from this region.’ Organized by CED and now in its...

by CytexOrtho | May 6, 2024 | In the News
MedTech Innovator’s mission “is to improve the lives of patients by accelerating the growth of companies that are transforming the healthcare system,” and CytexOrtho was excited to have been selected to participate! CytexOrtho was selected as one of...

by CytexOrtho | May 3, 2024 | In the News
From devicetalks.com: “In this episode of the DeviceTalks Weekly Podcast, Co-hosts Tom Salemi and Chris Newmarker interview Brad Estes, CEO of CytexOrtho, the winner of a pitch contest staged by AAOS. The company is developing implants that could serve as an...

by CytexOrtho | Feb 27, 2024 | In the News
We’re super proud of our CEO, Brad Estes, Ph.D., as he impressed the audience and judges alike to lead CytexOrtho to victory at the first inaugural AAOS OrthoPitch competition! Our sincere thanks to MCRA and American Academy of Orthopaedic Surgeons (AAOS) for giving...

by CytexOrtho | Feb 9, 2024 | In the News
CytexOrtho is excited to be selected as a finalist at the inaugural OrthoPitch contest next week at AAOS! CytexOrtho’s CEO, Dr. Brad Estes, will be battling it out with three other startups for the prizes and bragging rights at a brand-new technology competition for...







